Using Digital Tools to Track and Trace Health Commodities

Health commodities are essential components of well-functioning health systems. Governments must supply their citizenry with the right amount of these commodities at the right time and in the right place. However, this is not an easy task because supply chain processes are complex and highly interconnected. National supply chain operators must be able to track transactions and trace commodities from production to delivery to the people who need them, as illustrated in the figure below.
For countries to achieve complete traceability of health care commodities, end-to-end supply chain data management protocols must be in place. This requires manufacturers to label and barcode products so they can be traced. The most important factor for traceability is the presence of globally recognized unique identifiers of commodities. GSI is the most widely used system in the world for traceability. Most countries and manufacturers use GS1 with Global Trade Item Number (GTIN) serialization. Additionally, Global actors like WHO, GAVI, and UNICEF have introduced labeling standards on secondary and higher-level packaging for unique identification.
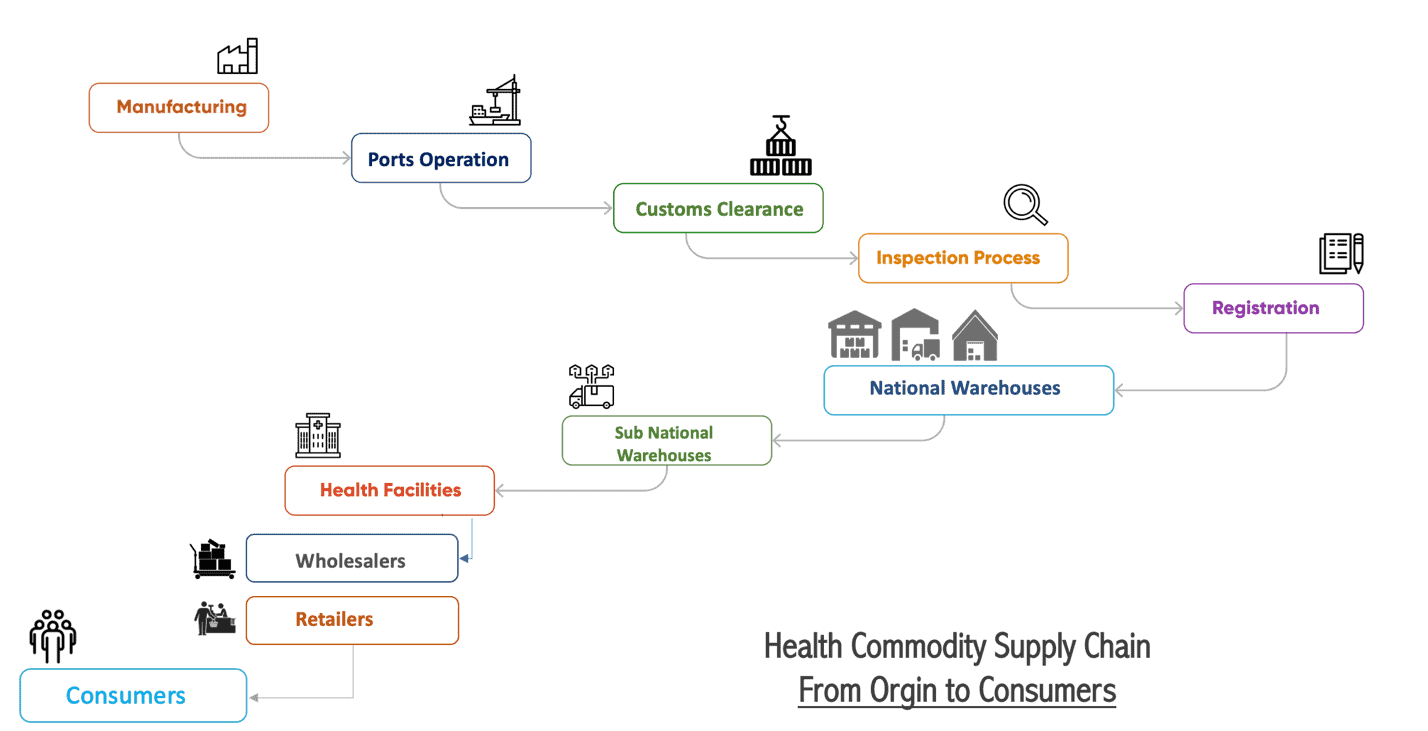
Ethiopia’s Health Commodity Supply Chain.
Benefits of traceability include improved public safety (by preventing counterfeit and falsified commodities); accountability (through transparency); and efficiency (by facilitating identification and resolution of supply chain hiccups).
What is Ethiopia doing on Health Commodity Traceability?
Ethiopia has significantly progressed in digitizing its supply chain. Its electronic logistics management information system (LMIS) encompasses the regulatory information system (from product registration to market release) and automation of supply chain operations at national, regional, and facility levels. The country also developed a mobile app that allows the public to verify the legitimacy of a product.
In mid-2021, USAID’s Digital Health Activity Ethiopia began supporting the government to develop an end-to-end track and trace system based on GS1 standards. Ethiopia has also endorsed the National Product Catalogue (NPC) to capture and authenticate the national product master data to serve all subsidiary systems within the wider LMIS. The following diagram illustrates the overall traceability approach that Ethiopia aspires to implement.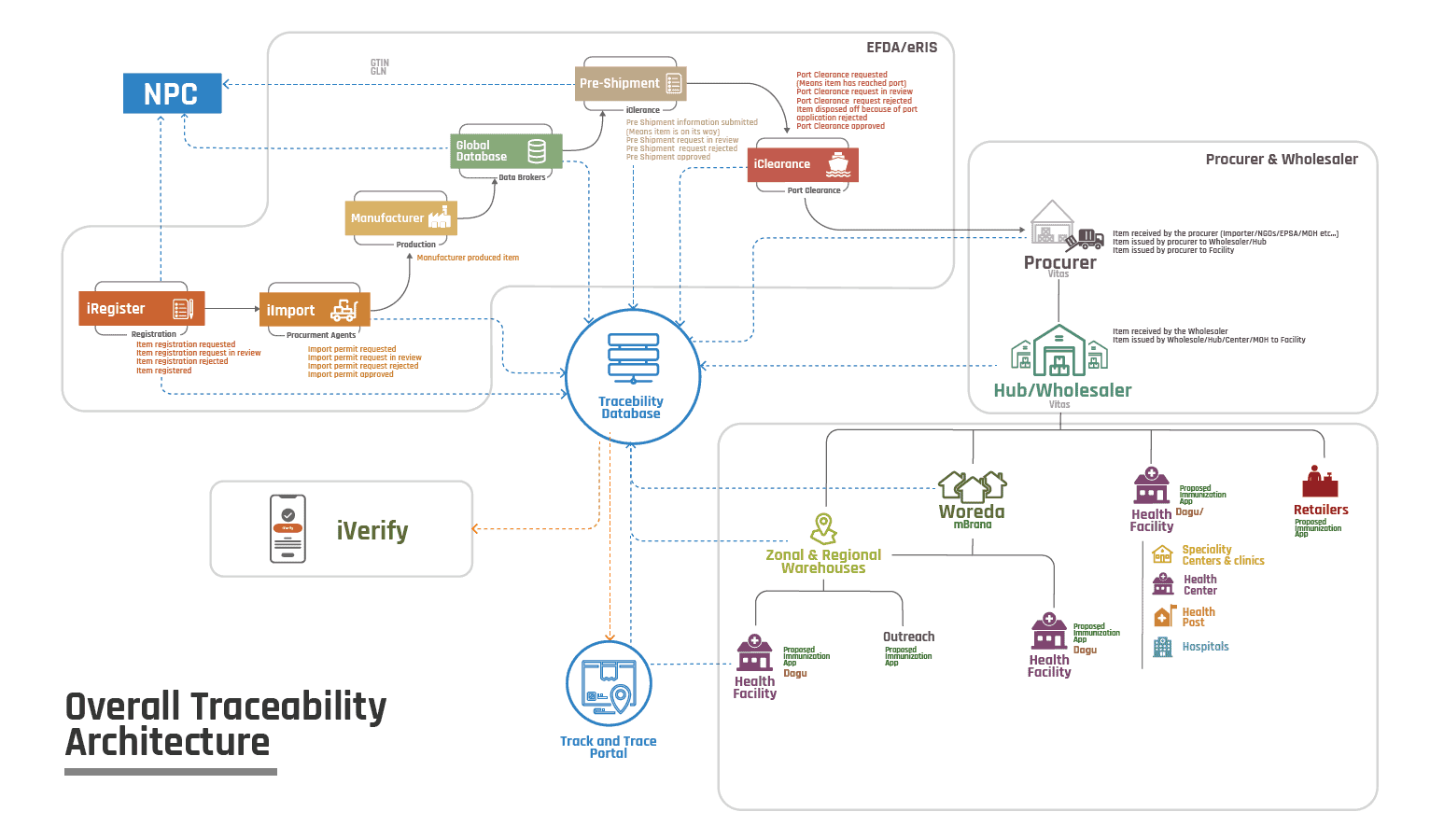
Ethiopia’s Health Commodity Traceability Architecture.
However, this initiative cannot succeed unless it is backed by policy and legislation. To this end, the Ethiopian Food and Drug Administration has developed and endorsed a traceability directive based on the GS1standard. Additionally, master data guidelines have been developed for capturing, labeling, and using standard pharmaceutical data, as have guidelines on barcode labeling, and acquisition and allocation of GTIN and Global Location Numbers. As these critical pieces fall into place, Ethiopia is advancing its realization of a health commodity track-and-trace system that ensures accountability, efficiency, and most importantly, public safety.
Article by:

Digitalization Director, USAID Digital Health Activity
